Which Best Describes a Weak Acid
Examples of weak acids includes acetic acid hydrogen sulfide formic acid hydrofluoric acid etc. Any suggestion would be appreciated.

Reactions In Aqueous Solutions Ppt Download
D a bicarbonate io n.

. An acid is a substance that donates H ions in a reaction. It always loses less H when dissolved in water. It contains covalent bonds.
Buffers are weak acid and conjugate base pairs that resist changes in the pH of a solution. Which statement always accurately describes acids and bases a the conjugate base of a weak acid is a strong base b the conjugate acid of a. It must be of low solubility and completely ionized Homework Equations The Attempt at a Solution Homework Statement.
The initial pH before the addition of any strong base is higher or less acidic than the titration of a strong acid. Buffers promote pH changes of a solution by contributing hydronium ions to the solution. Partially ionizes and is corrosive to metals D.
An acid that dissociates very slightly in a water solution e. 1 C 2 H 4 O 2 a q O H a q C 2 H 3 O 2 a q H 2 O l In this reaction a buret is used to administer one solution to another. PKa values change over the course of a titration.
The antacid is basic and. An acid or a base dissolved in water breaks down into a positive and a _____ ion. A dilute strong acid c.
I encountered a bit of a dilemma in picking the correct answer. For bases the higher the pH the more basic a substance is. 2018-02-05 12.
100 9 ratings Transcribed image text. C is only partially ionised in aqueous solution. 82 Choose the answer that best describes HCO 3 -.
Anything lower than 7 is acidic and anything higher than 7 is basic. Rate of reaction is slower. It is present in large quantities in a solution.
Conducts electricity and tastes sour C. See answers 2 Best Answer. It may be very soluble but only partly ionized.
Note that a pH of 7 is considered neutral. It must be very soluble and completely ionized D. Which of the following best describes a weak acid.
Turns blue litmus paper red B. Which statement best describes these two acids. The solution administered from the buret.
In the reaction the acid and base react in a one to one ratio. Which statement best describes a weak acid. On the other hand the reaction arrow for a weak acid ionizing in water is a double arrow indicating that both the forward and reverse reactions occur at equilibrium.
Pyridine is a weak base with the formula C5H5N. This is because the anion of the weak acid becomes a. HCl completely dissociates in water while HC2H3O2 does not.
An acid that dissociates completely in solution c. A substance in a solution that releases hydrogen ions and lowers the pH. It resists dissociating into ions in solution.
D reacts slowly with magnesium ribbon. A weak acid is best described as one that. At similar concentration weak acids have higher pH value than strong acids.
Apsiganocj and 11 more users found this answer helpful. Which best explains the relationship. There is a sharp increase in pH at the beginning of the titration.
Which best describes the pH at the equivalence point of a titration of a weak acid with a strong base. Which best describes the antacids effect. A weak acid is an acid that is slightly dissociated into its ions in a solution.
An acid that is not very strong b. In aqueous solution acetic acid partially dissociates according to the following reaction. An acid with a very low concentration d.
Weak acids do not completely ionize. Its 010 M solution will have a pH 100 B. It possesses low conductivity due to the presence of fewer unpaired atoms.
It is present in low quantities in a solution. CH3COOH CH3COO- H Use the Ka equation. Ionization of Weak Acids.
An acid that ionizes very slightly in a water solution. Hydrochloric acid HCl and acetic acid HC2H3O2 are both acids. Weak acids ionize partially in an aqueous solution.
Which statement best describes what it means when something is classified as a strong acid. The pKa value depends on the concentration of the molecule present. Weak acids are not highly concentrated.
The pKa value depends on the pH. 83 Select which reactions will usually be irreversible regarding chemical equilibrium in human bodies. An acid in a dilute solution b.
It easily dissociates into ions in solution. An acid in a concentrated solution d. Group of answer choices less than zero between 0 and 7 close to 70 greater than 14 between 7 and 14.
The reaction symbol for a strong acid ionizing in water is a simple arrow facing from left to right. There are several characteristics that are seen in all titration curves of a weak acid with a strong base. B has a pH only slightly less than 7.
Fully dissociates and establishes an equilibrium in solution. The reaction of the weak acid acetic acid with a strong base NaOH can be seen below. Acetic acid is a weak acid with the formula CH3COOH the Ka for acetic acid is 176 x 10-5.
HCl is a strong acid HC2H3O2 is a weak acid. Buffers are solutions containing a strong acid and a weak base resulting in neutralization. Which of the following best describes a weak acid A.
Which statement best describes a weak acid. A a weak acid B a proton donor C common in the liver D a bicarbonate ion Answer. Apple juice has a lower pH than black coffee.
Choose the answer that best describes hco 3 a a weak. An acid that dissociates only slightly in solution. Weak acids are not dangerous since they are weak.
Which statement best describes a weak acid. Science Chemistry QA Library Which of the following best describes Buffers. A contains a low concentration of an acid.
Part 1 1 point See Periodic Table See Hint Which best describes the pka of a weak acid. A pH of 8 describes a weak base. Weak acids do not conduct electricity.
At equilibrium the weak acid its conjugate base and the hydrogen ion are all.
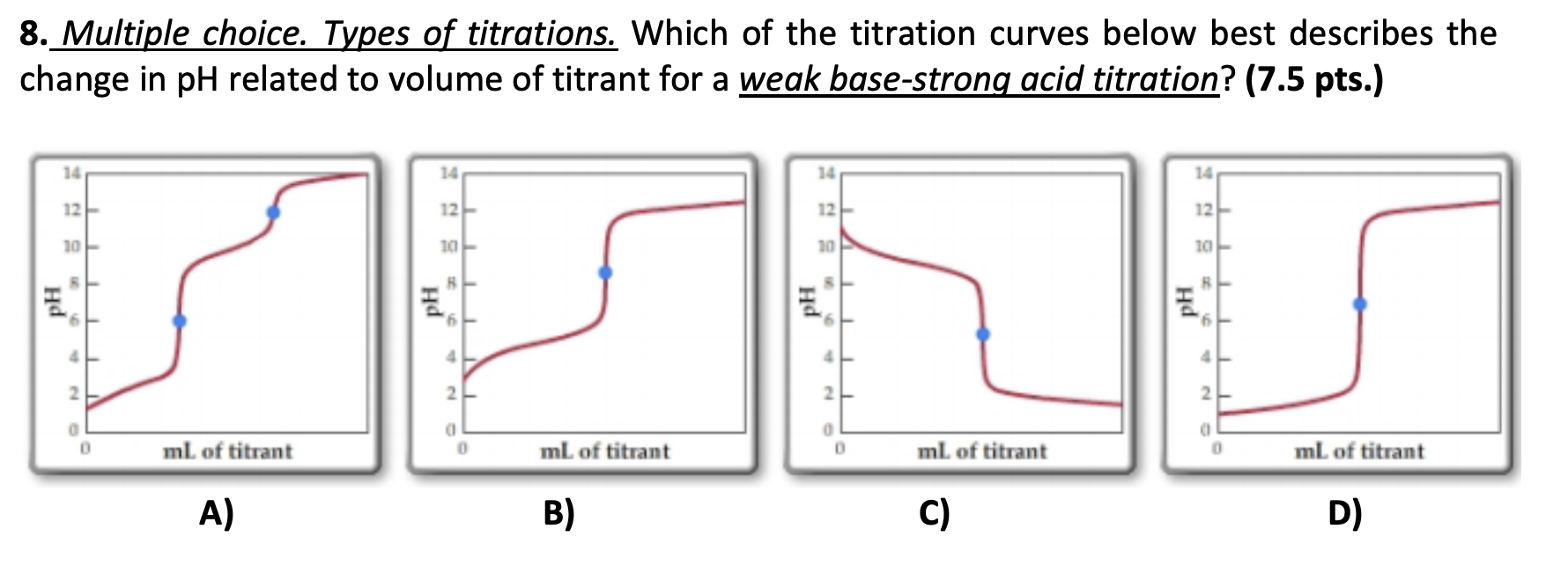
Solved 8 Multiple Choice Types Of Titrations Which Of The Chegg Com

Solved Part A As You Saw In The Video The Ph Of A 0 1 M Chegg Com
Comments
Post a Comment